INVESTIGATIVE REPORT: Health Canada and the Pfizer-BioNTech COMIRNATY Deception
How the Government of Canada misled Canadians on the Regulatory Status of the Pfizer-BioNTech Comirnaty Covid-19 Vaccine as proven "Safe and Effective"
Above screenshot from “Will covid-19 vaccines save lives? Current trials aren’t designed to tell us” (Published 21 October 2020), by Peter Doshi, associate editor of the British Medical Journal (BMJ).
This is Part One of a three-part Investigative Report, as follows:
Part One:
Reviews published claims by Health Canada that the Pfizer-BioNTech COMIRNATY Covid-19 vaccine has been “approved” and “proven safe and effective”, and shows that the Government knows these claims are untrue.
Provides details of the Health Canada - Regulatory Decision Summary - to authorize the use of the Pfizer-BioNTech COMIRNATY Covid-19 vaccine;
Outlines specifics on the regulatory classification assigned by the Minister of Justice to the Pfizer-BioNTech COMIRNATY Covid-19 vaccine as a New Drug under Division 8, section C.08.001, of the Food and Drug Regulations, and the implications of this classification.
Provides the definition of “Fraud” in the Criminal Code of Canada, and
Presents an analysis conducted by researchers at Columbia University on “COVID vaccination and age-stratified all-cause mortality risk”.
Part Two and Part Three of this Investigative Report have been published separately.
Part Two: documents how Canadian regulators may rely on the US FDA (“the FDA”) as a “foreign regulatory authority” in the Pre-positioning of Designated COVID-19 Drugs, and outlines the content and implications of two (2) confusing letters the FDA wrote to Pfizer Inc., and to BioNTech GmbH on August 23, 2021, with respect to the authorization (re: BioNTech & COMIRNATY) and licensing (re: COMIRNATY) of their Covid-19 designated drugs.
Part Three: Epidemiology and the Politics of Deception, provides an overview of the known Epidemiology of Covid-19 and the military grade Information Operation applied against an unwary public.
Proven Safe and Effective? Division 8 of Canada’s Food and Drug Regulation — New Drugs
As with its essentially identical while legally distinct precursor BioNTech (see Part Two), the Pfizer’s COMIRNATY Covid-19 designated drug (aka ‘vaccine’) is only authorized (def: to grant permission) for use in Canada.
In fact, this Covid-19 designated drug has not been approved (def: to sanction officially), nor, by definition of its regulatory classification in Canada, has it been established as safe and effective.
[Note, a manufacturer of an (emergency) authorized drug is generally protected by a blanket liability shield, but can lose that liability shield once the drug is fully approved for sale — see Part Two of this report for further details.]
This has significant implications for parents of children under age 19, as well for employers who have imposed vaccine mandates on workers; who may have relied on Health Canada’s published claims that it had '‘approved” a number of Covid-19 designated drugs (‘vaccines’).
For details on the Pfizer-BioNTech COMIRNATY COVID-19 vaccine regulatory classification in Canada in contrast to what has been claimed, see the following three (3) screenshots and related documents found in the various hyper-links.
1) Below is the screenshot below from a Health Canada webpage concerning the Pfizer-BioNTech COMIRNATY COVID-19 vaccine, wherein Health Canada declares that:
All COVID-19 vaccines authorized in Canada "“are proven safe and effective and of high quality”,
The “Status: of the Pfizer-BioNTech COMIRNATY COVID-19 is: “Approved by Health Canada”, and
The has “vaccine” has also been “Approved for: Age 5 and older”.
Figure 1: Government of Canada “ALL COVID-19 vaccines in Canada are proven safe, effective”
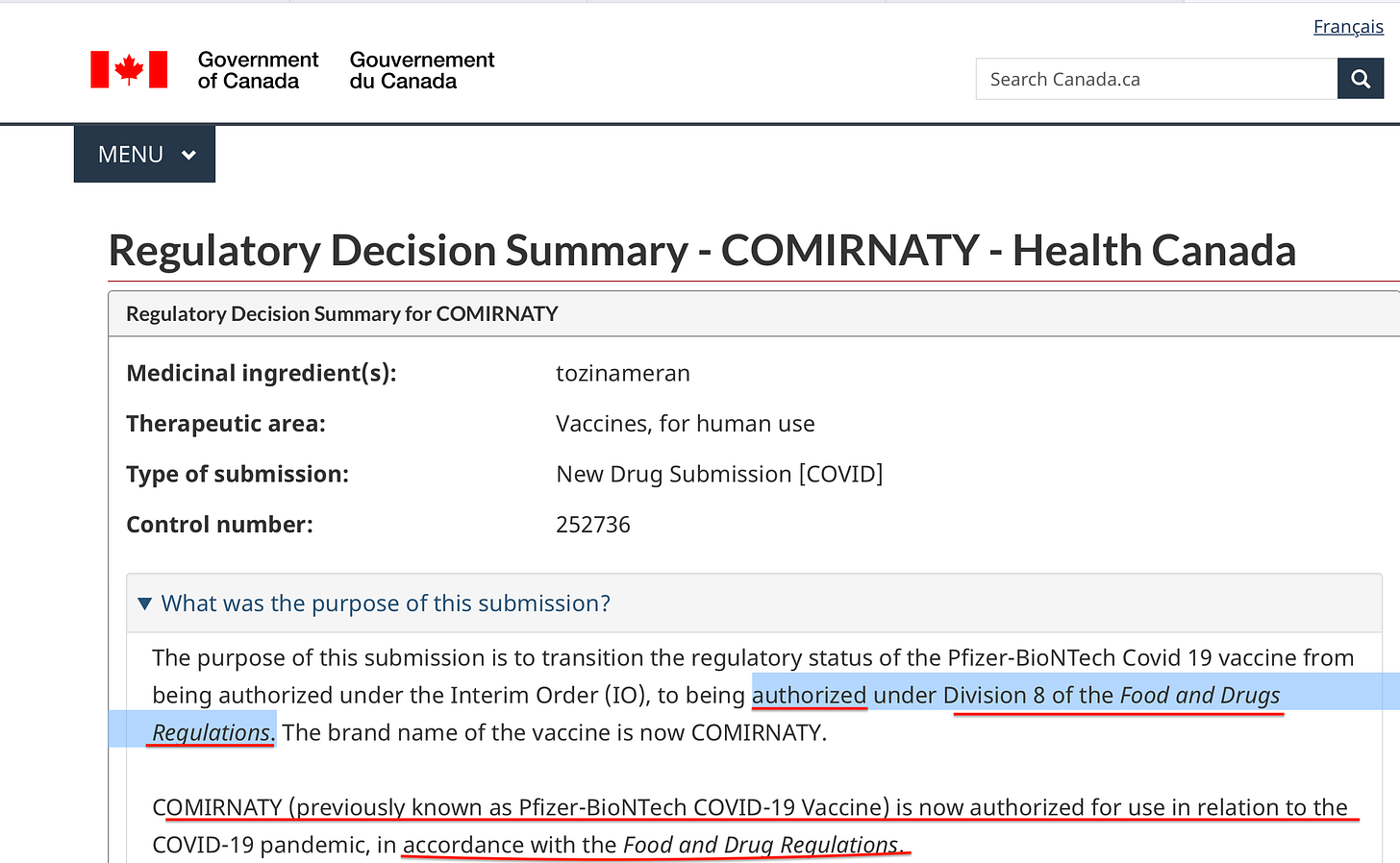
2) However, in the screenshot shown below taken from Health Canada’s Regulatory Decision Summary - COMIRNATY — Health Canada, we can clearly see that it states that this Covid-19 vaccine has (only) been “authorized”, and under Division 8 of the Food and Drug Regulations, an obscure section of this voluminous ten-part 1,154 page document.
Figure 2: Regulatory Decision Summary - COMIRNATY - Health Canada (Division 8)
But to what does Division 8 of the Food and Drug Regulations actually relate? Read on.
3) Division 8 of the Food and Drug Regulations concerns New Drugs, and at Section C.08.001 of the Regulations it states that the classification of a “New Drug” refers to a drug:
(a) … that has not been sold as a drug in Canada for sufficient time and in sufficient quantity to establish in Canada the safety and effectiveness of that substance for use as a drug.
Therefore, by its very definition, a ‘New Drug’ means a drug that had not been shown to be safe and effective. Below is a screenshot of section C.08.001 in full.
Figure 3: Division 8 of the Food and Drug Regulations - New Drugs
Division 8 of the Food and Drug Regulations goes on to define a “designated Covid-19 Drug" as a new drug:
for which the purpose and conditions of use recommended by the manufacturer relate to COVID-19;
Therefore, Health Canada’s published claims that the Pfizer-BioNTech COMIRNATY COVID-19 vaccine has been “approved” and “proven safe and effective” is in direct conflict with this designated Covid-19 drugs actual regulatory status.
To restate, we can clearly see that the Pfizer-BioNTech COMIRNATY COVID-19 vaccine has only been authorized for use secondary to its classification as a “New Drug” under section C.08.001 in Division 8 of the Food and Drug Regulations — meaning, by definition, the safety and efficacy of this designated Covid-19 drug has not been established. To claim otherwise elsewhere would appear to be misleading, at best.
Can governments or employers in Canada mandate or otherwise coerce the uptake of a drug when its safety and efficacy has not been established? In my view, as an insurance and risk management professional with over three decades in medical-legal practice, they most certainly cannot. The liability implications here could be staggering.
So what is really going on? Have Canadians been misled so that Proof of Vaccination Public Health Orders could be plausibly deployed by Provincial Health Officers, vaccine mandates imposed by both governments as well as a great many large employers, and these ‘New Drugs’ even marketed directly to children? Was this part of a coordinated effort to increase the uptake of an unproven drug by deploying the type of manipulative techniques recommended by the World Health Organization?
If we were to attempt to untangle things we might begin by asking: would the drug manufacturer’s liability shield (see Part Two) be at risk if fraud is found? After all, fraud, for which Big Pharma has plead guilty in the past, eviscerates all contracts.
Pfizer has paid out billions in civil lawsuits, including $2.3 billion that Pfizer and a subsidiary agreed to pay out for the largest healthcare fraud settlement in the history of the US Justice Department.
Section 380 of the Criminal Code of Canada — Fraud, states:
(1) Every one who, by deceit, falsehood or other fraudulent means, whether or not it is a false pretence within the meaning of this Act, defrauds the public or any person, whether ascertained or not, of any property, money or valuable security or any service,
(a) is guilty of an indictable offence and liable to a term of imprisonment not exceeding fourteen years, where the subject-matter of the offence is a testamentary instrument or the value of the subject-matter of the offence exceeds five thousand dollars; or
(2) Affecting public market
Every one who, by deceit, falsehood or other fraudulent means, whether or not it is a false pretence within the meaning of this Act, with intent to defraud, affects the public market price of stocks, shares, merchandise or anything that is offered for sale to the public is guilty of an indictable offence and liable to imprisonment for a term not exceeding fourteen years.
Pfizer’s own trial data for its Covid-19 vaccine, which is starting to be released in response to a Freedom of Information Act (FOIA) request to the FDA, do nothing to assuage safety concerns. Quite the contrary. We have learned that, cumulatively, between December 1, 2020, and February 28, 2021 — a period of just 2.5 months — Pfizer received 42,086 injury reports, including 1,223 fatalities.1
This is an especially important issue given that the FDA is currently in a US Federal Court (which Pfizer attempted to join) trying to prevent a group of public health officials and medical professionals from having further access to the FDA's Pfizer Covid-19 vaccine licensing records via FOIA. An issue that the senior editor of the British Medical Journal (BMJ) weighed-in on in a recent editorial: Covid-19 vaccines and treatments: we must have raw data, now (for a detailed review, see end note 2).
Here’s another riddle to consider, a report from Reuters on February 5, 2022 — Pfizer drops India vaccine application after regulator seeks local trial — stated that the drug company would rather miss out on billions of dollars of guaranteed profit selling its mRNA Covid-19 vaccines in India than submit to an independent local trial to:
determine if the vaccine is SAFE and generates an immune response in its citizens.
Let this sink in.
Perhaps Pfizer and the FDA are hiding safety data of much more significance than is generally known? If so, a number of recent studies may provide some clues about what is being hidden.
In an analysis published in October 2021 by researchers at Columbia University — COVID vaccination and age-stratified all-cause mortality risk — the authors stated:
Accurate estimates of COVID vaccine-induced severe adverse event and death rates are critical for risk-benefit ratio analyses of vaccination and boosters against SARS-CoV-2 coronavirus in different age groups. However, existing surveillance studies are not designed to reliably estimate life-threatening events or vaccine-induced fatality rates (VFR).
According to the authors:
Comparing our age-stratified VFRs [vaccine-induced fatality rates] with published age-stratified coronavirus infection fatality rates (IFR) suggests the risks of COVID vaccines and boosters outweigh the benefits in children, young adults and older adults with low occupational risk or previous coronavirus exposure.
We discuss implications for public health policies related to boosters, school and workplace mandates, and the urgent need to identify, develop and disseminate diagnostics and treatments for life-altering vaccine injuries.
If “existing surveillance studies are not designed to reliably estimate life-threatening events or vaccine-induced fatality rates”, is it not disingenuous for public health officials to claim that “no safety issues were detected” with respect to the administration of these New Drugs?
See below “Model-estimated deaths attributed to Covid Vaccination” in the US for Jan-Aug 2021, from the analysis conducted by researchers at Columbia University. Please, note the highlighted boxes and the Totals column.
Figure 4: from COVID vaccination and age-stratified all-cause mortality risk (October 2021)
Meanwhile, Sweden has decided against recommending COVID vaccines for children aged 5-11, arguing that the benefits did not outweigh the risks. Not so Health Canada.
"With the knowledge we have today, with a low risk for serious disease for kids, we don't see any clear benefit with vaccinating them," a Sweden Health Agency official told a news conference.
As noted in the preamble, in Part Two of this Investigative Report — The FDA, a foreign regulatory authority in Canada? — we will review the section of Division 8 of the Food and Drug Regulations that allows Health Canada, in the Pre-positioning of Designated COVID-19 Drugs, to rely on:
an application (that) has been submitted to a foreign regulatory authority to authorize the sale of the designated COVID-19 drug.
Which will be followed by a detailed analysis of the US FDA’s authorization and licensing of the COMIRNATY Covid-19 vaccine, in response to a submission by Pfizer-BioNTech of a Biologic License Application (BLA) for this designated Covid-19 drug.
End Notes:
1 My dictionary defines “established” in the present context as: shown to be true or certain by determining the facts, and “proven” as: demonstrated by evidence to be true. In other words, these are synonyms: they mean the same thing.
2 Re: BMJ https://doi.org/10.1136/bmj.o102: Covid-19 vaccines and treatments: we must have raw data, now
The British Medical Journal (BMJ) is a weekly peer-reviewed medical trade journal published by the trade union of the British Medical Association, and has editorial freedom from the British Medical Association. In an editorial published on January 19, 2022 the BMJ called for the release of the raw data from the trials used to first authorize the Covid-19 vaccines for (emergency) use, and now mandate vaccine uptake.
Excerpt from the BMJ editorial:
“Today, despite the global rollout of COVID-19 vaccines and treatments, the anonymized participant-level data underlying the trials (used by governments to first authorize the use of the vaccines and now increasingly mandate their uptake) for these new products remain inaccessible to doctors, researchers, and the public—and are likely to remain that way for years to come,”
“This is morally indefensible for all trials, but especially for those involving major public health interventions.”
BMJ also accused pharmaceutical companies of “reaping vast profits without adequate independent scrutiny of their scientific claims,” pointing to Pfizer, whose COVID vaccine trial was “funded by the company and designed, run, analyzed, and authored by Pfizer employees.”
New York-headquartered Pfizer still holds that trial data and has indicated that it won’t begin considering requests for such data until May 2025 – 24 months after the primary study completion date of May 15, 2023, which is listed on ClinicalTrials.gov as well as in Part Two of this report.
Meanwhile, The Food and Drug Administration (FDA) had asked a judge to give it 75 years to produce all the data concerning the Pfizer and BioNTech vaccine.
However, a judge recently ordered that the FDA make public 12,000 pages of the data it used to make decisions regarding approvals for the Pfizer/BioNTech COVID-19 vaccine by the end of the month. The FDA must also release Pfizer’s vaccine data at a rate of 55,000 pages a month until all of the requested pages are public.
“We are left with publications but no access to the underlying data on reasonable request,” BMJ said.
“This is worrying for trial participants, researchers, clinicians, journal editors, policymakers, and the public. The journals that have published these primary studies may argue that they faced an awkward dilemma, caught between making the summary findings available quickly and upholding the best ethical values that support timely access to underlying data. In our view, there is no dilemma; the anonymized individual participant data from clinical trials must be made available for independent scrutiny.”
BMJ added that regulators are not there to “dance to the tune of rich global corporations and enrich them further” but to protect the general public’s health and for that reason, they said, we need “complete data transparency for all studies, we need it in the public interest, and we need it now.”
Also of note (H/T Kelly Brown @rubiconcapital), Pfizer recently added new and peculiar items deep in its business risk disclosures re: clinical trial data, in its Q4 earnings report for 2021, concerning: “...risks associated with...further information regarding the quality of pre-clinical, clinical or safety data, including by audit or inspection;”
And "...challenges related to public confidence or awareness of our COVID-19 vaccine … including … CONCERNS ABOUT CLINICAL DATA INTEGRITY and prescriber and pharmacy education;"
“Fact Checkers” noted that Pfizer didn’t determine cause and effect, only that more than 1,000 vaccinated people died in the first three months after the vaccine's emergency use authorization from the FDA on December 11, 2020.
Pfizer’s document spells out its limitations:
Pfizer's safety database contains cases of AEs (Adverse Events) reported spontaneously to Pfizer, cases reported by the health authorities, cases published in the medical literature, cases from Pfizer-sponsored marketing programs, non-interventional studies, and cases of serious AEs reported from clinical studies regardless of causality assessment.
What we don’t know about the “cause and effect” of the Adverse Event’s reported post-vaccination is precisely why researchers require Pfizer’s raw data.